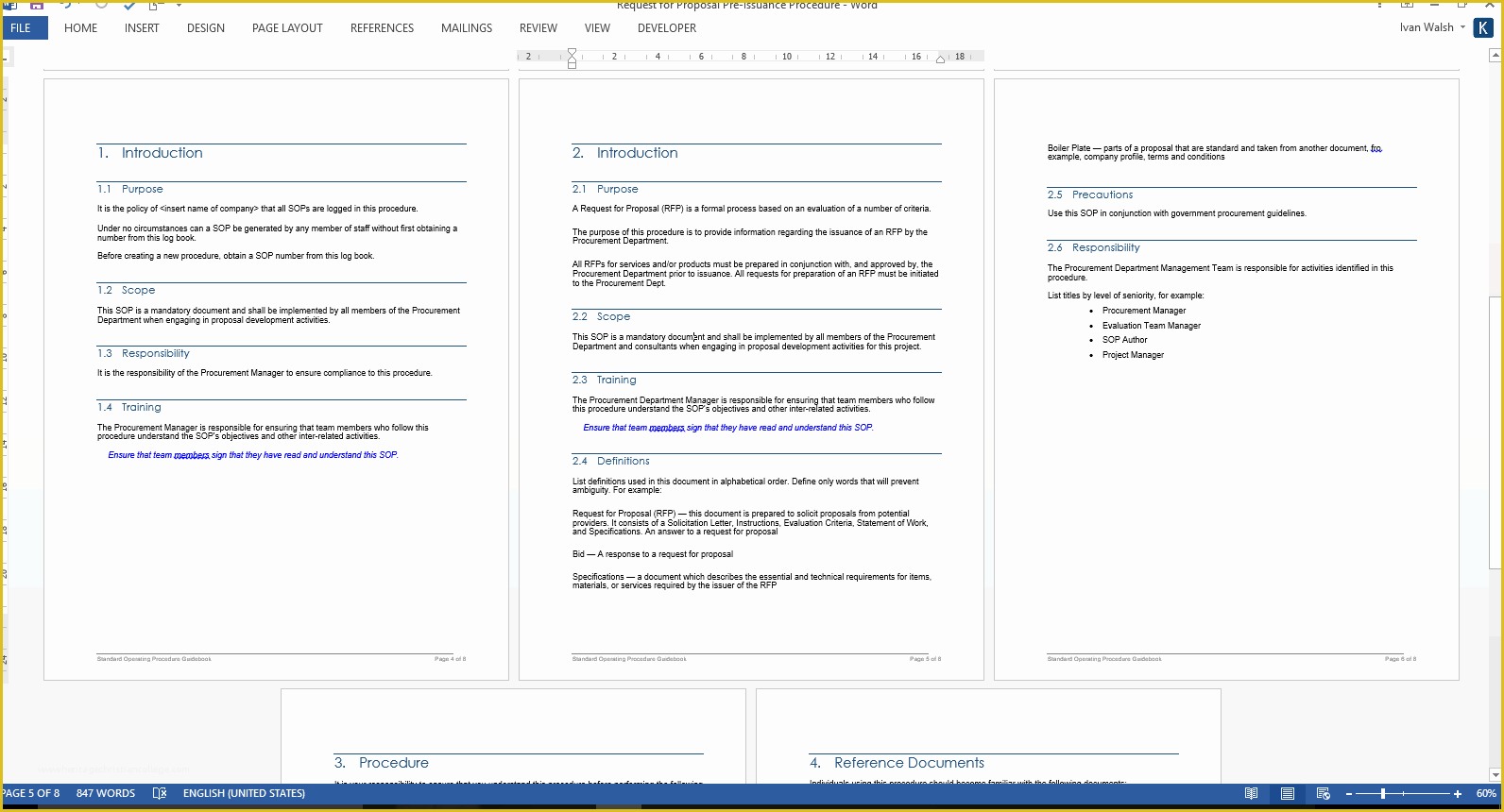
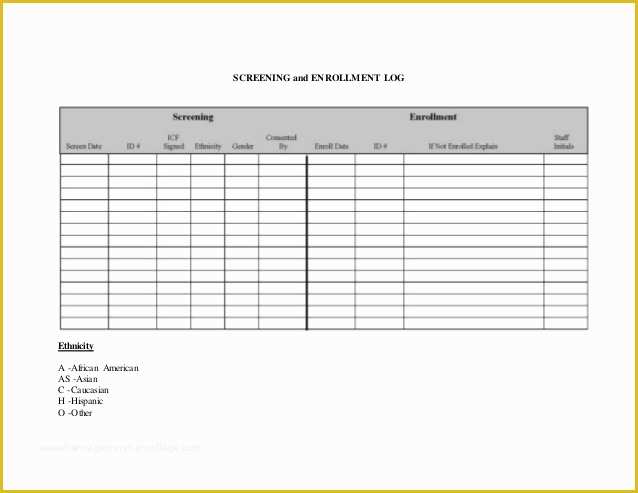
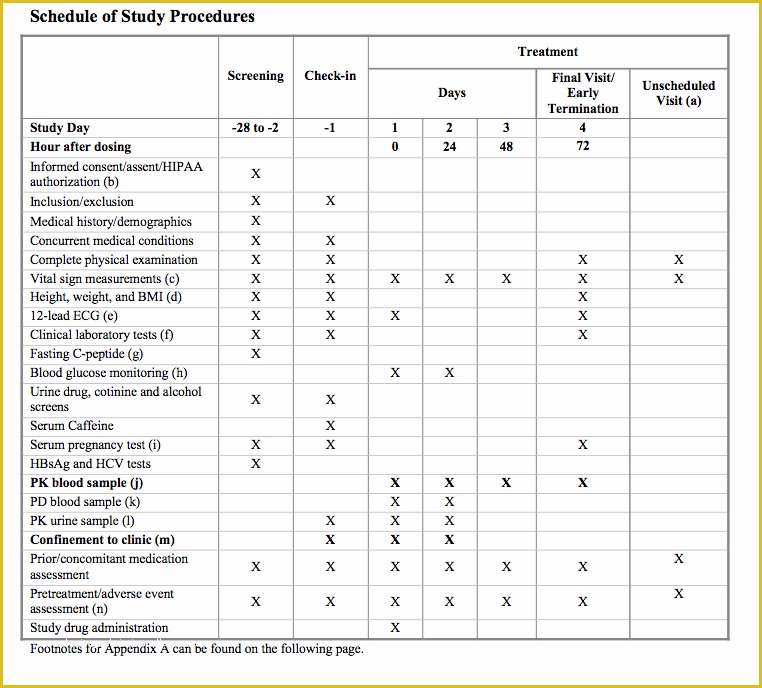
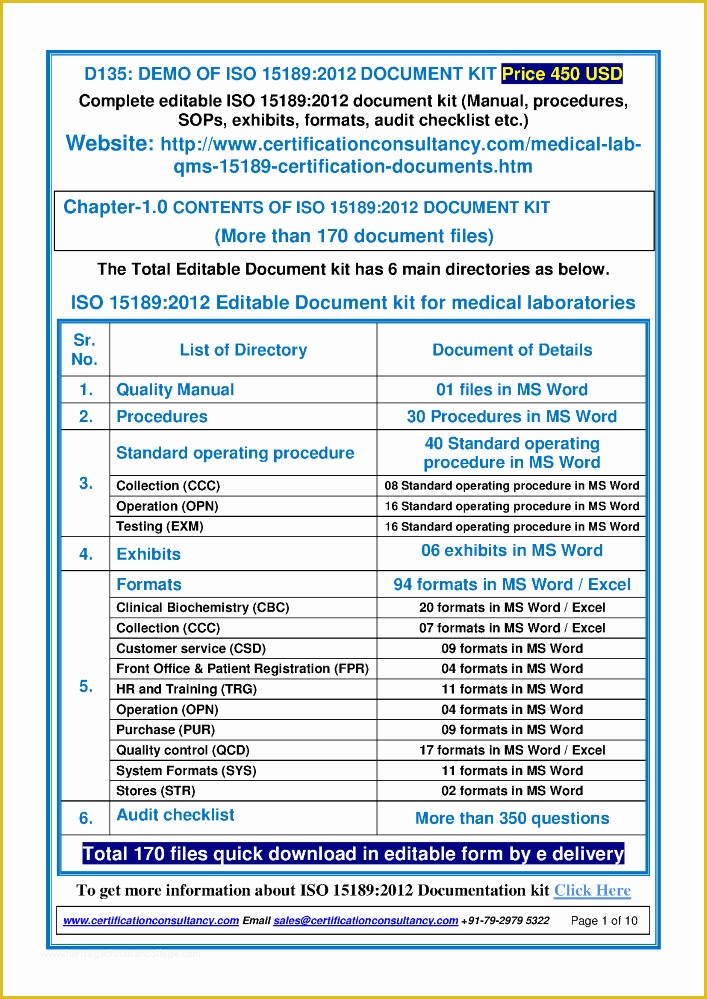
Standard Operating Procedure SOP Template User Guide Data Retention Policy Template Orientation for New Clinical Research PERSONNEL Module 2 36 Page Standard Operating Procedure SOP Template MS Clinical Trial Protocol Template Ich Templates Resume .
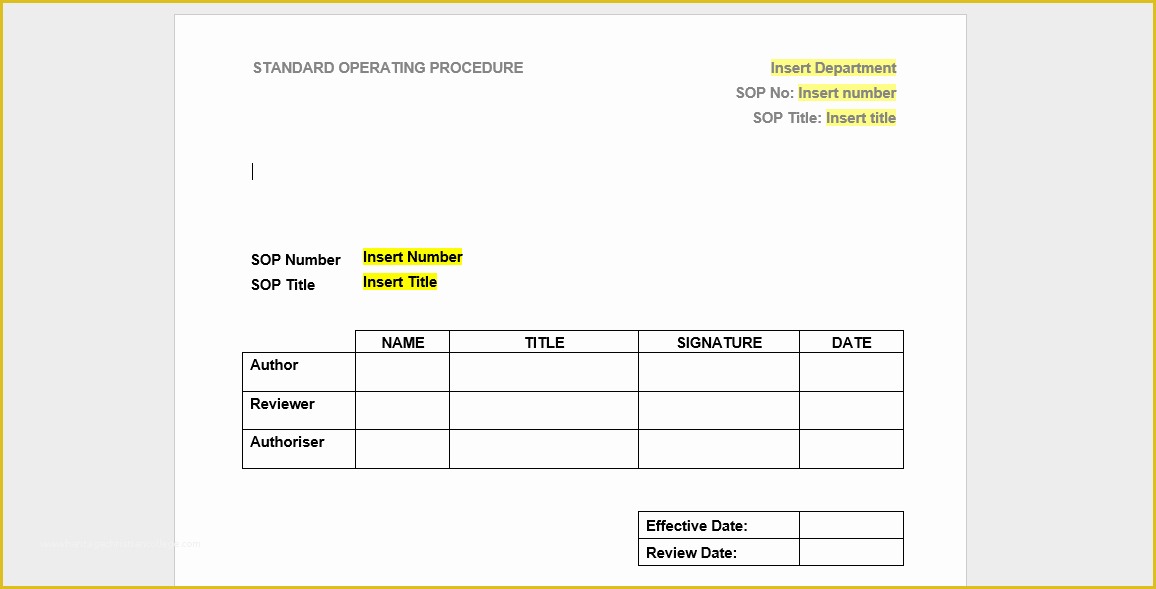
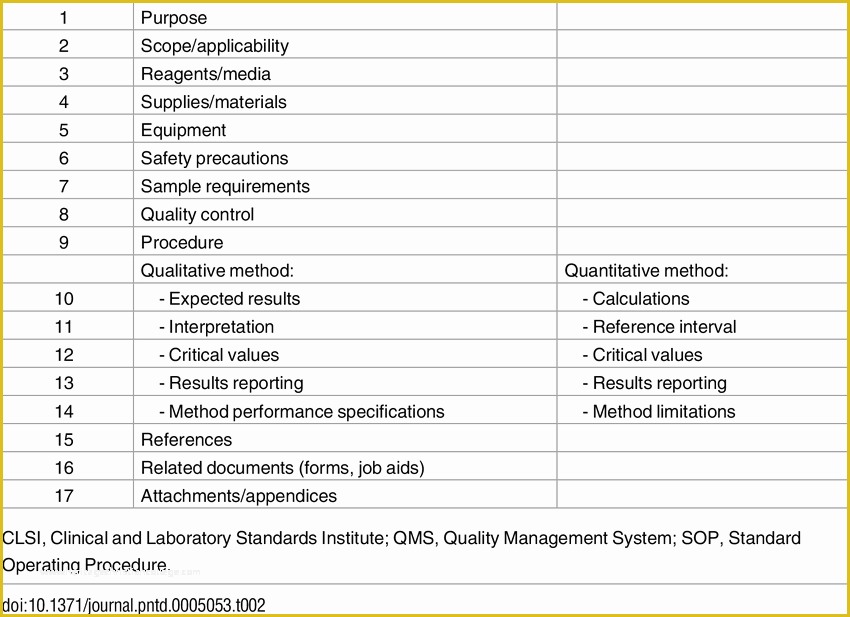
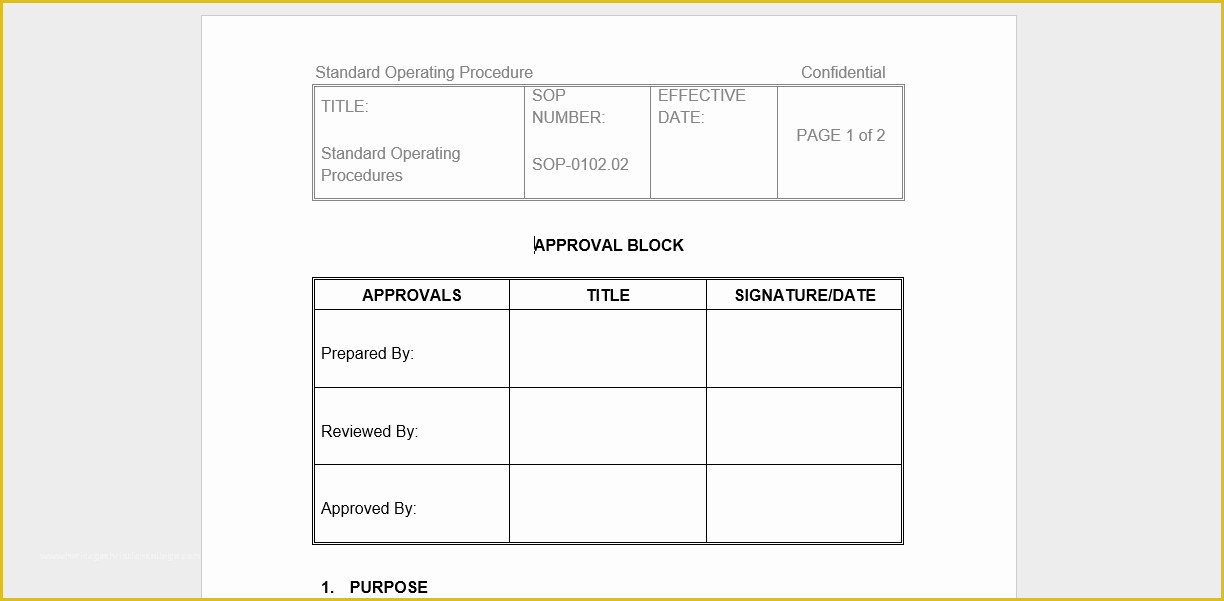
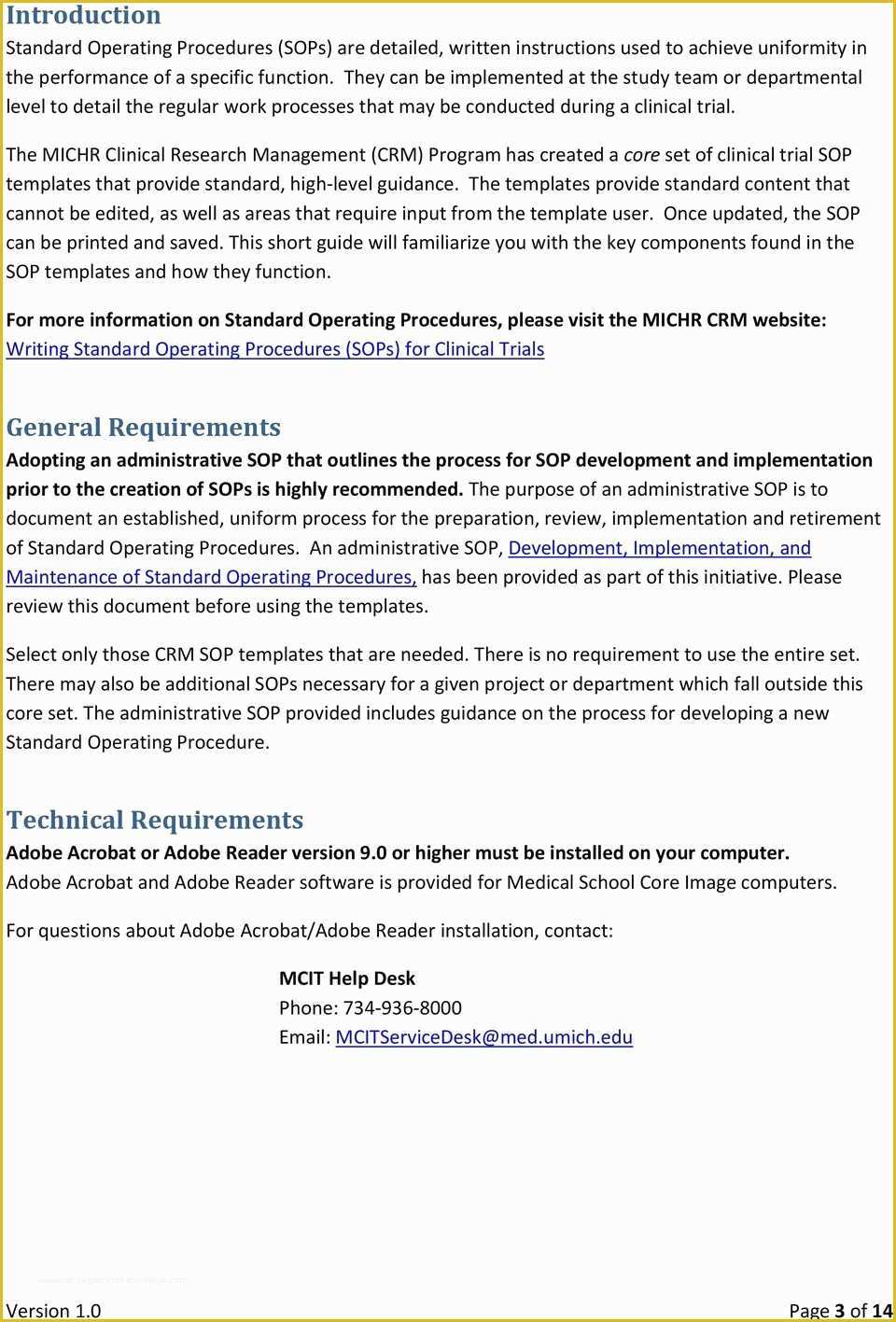
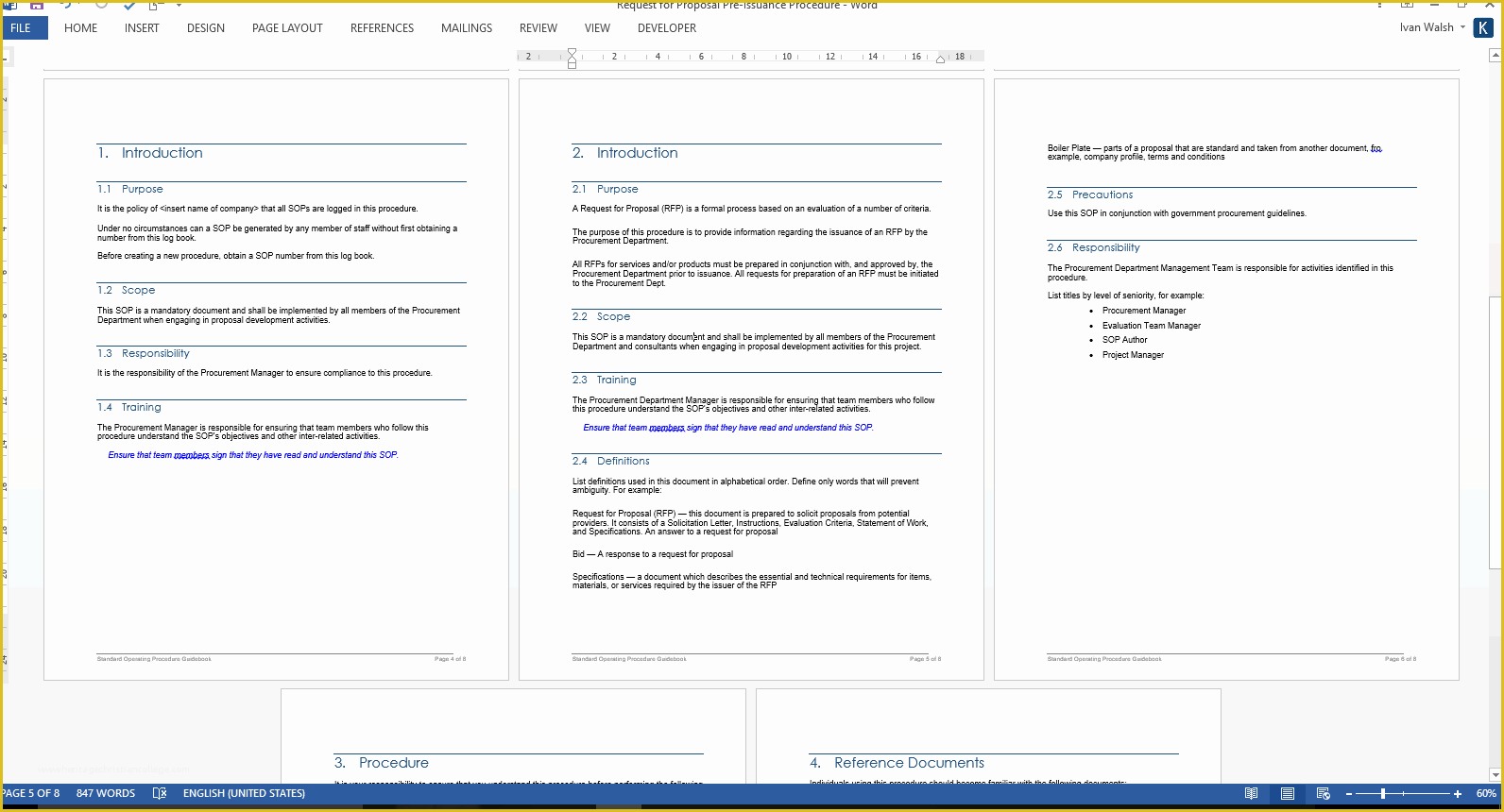
article downloadable templates and tools for clinical wel e to global health trials tools and templates library please note that this page has been updated for 2015 following a quality check and review of the templates and many new ones have been added sops and templates sops and templates standard operating procedures sops are detailed written instructions which must be followed when performing certain tasks they are an essential source for municating to researchers the agreed defined methodology which must be followed to ensure consistency between researchers and in multi centre stu s the consistency between individual research sites 45 free sop template sample templates the best way to design your own standard operating procedure would be to use a free sop template as the framework however if you wish to make your own standard operating procedure without using a template you can follow the steps below sop writing for clinical research iths sop writing for clinical research write down what you do do what is written down mandy vick research pliance monitor regulatory support & bioethics core standard operating procedures for clinical research standard operating procedures for clinical research departments ashley nichole kee w riting and reading about the need for stan dard operating procedures sops is almost as exciting as creating implementing and tracking a set of sops do not worry there are many consultants and possibly members of a practice’s current staff that have the ability to create a set of sops for conducting standard operating procedures for clinical trials sops standard operating procedures for clinical trials sops dghi is pleased to share these documents with others who are working in resource limited locations although most of these sops were developed for aids clinical trials many of these documents can be modified and extrapolated to meet your specific project needs clinical research gcp sops all clinical research investigators and staff are accountable for following good clinical practice gcp guidelines when conducting human subjects research the college of medicine fice of research has developed gcp clinical research standard operating procedure sop templates which provide detailed written instructions for conducting clinical research at the investigational site standard operating procedures hub clinical research in clinical research sops help define the group’s e g unit division department institution etc standard practices and daily processes conducted to assure execution of research tasks in accordance with institutional state and federal guidances sops a must for sites sops are intended to support pliance with laws and regulations that govern the conduct of clinical research it is not necessary to document every service you provide
clinical research t bingen, clinical research assistant, clinical research services, clinical researcher, clinical research scientist biothec germany, clinical research center, clinical researcher job description, clinical research unit, clinical research limited and its affiliates, clinical research mannheim,
clinical research ,