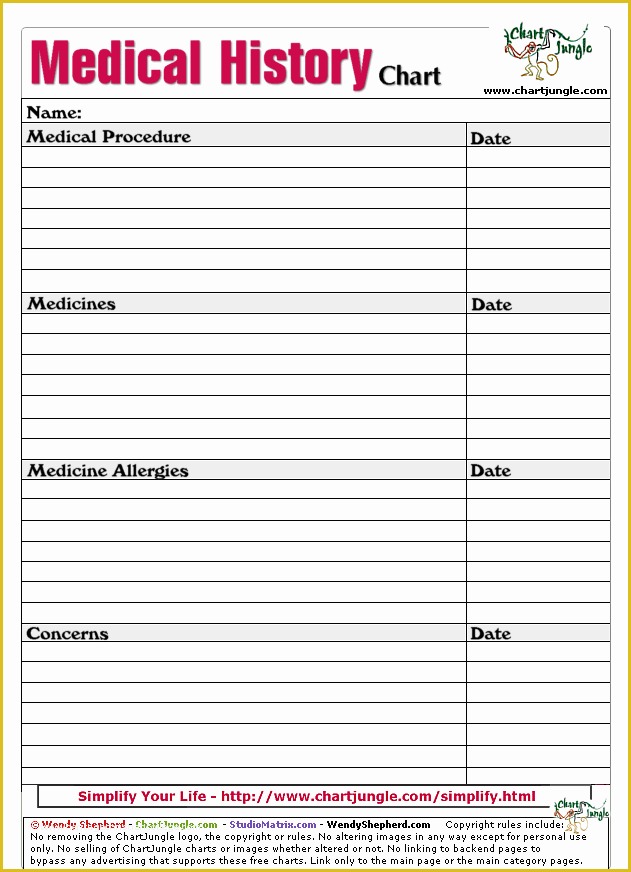
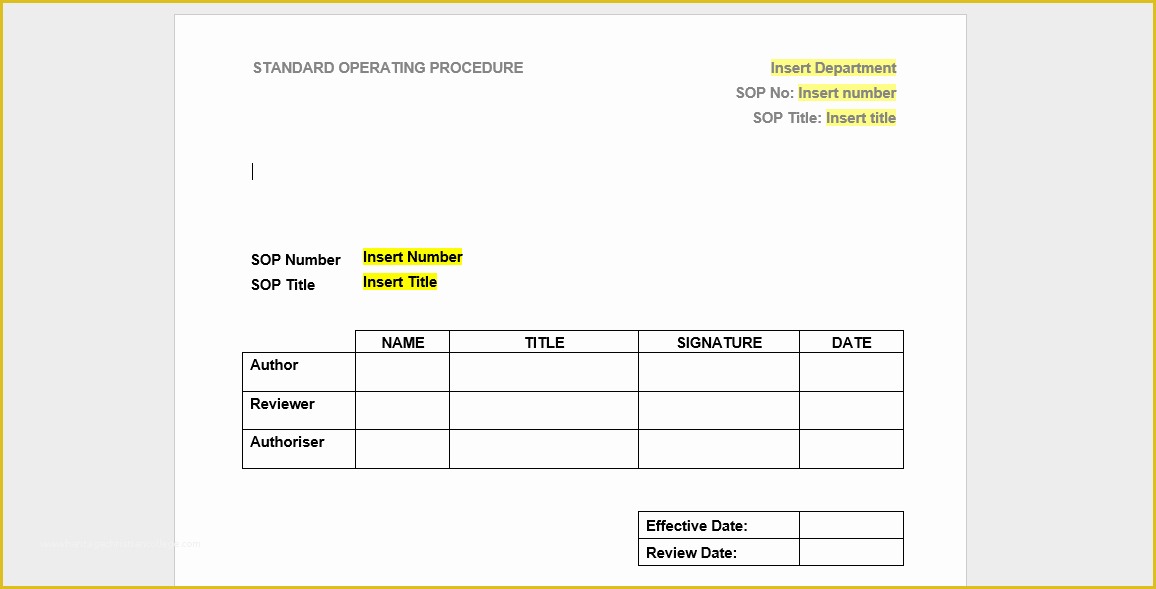
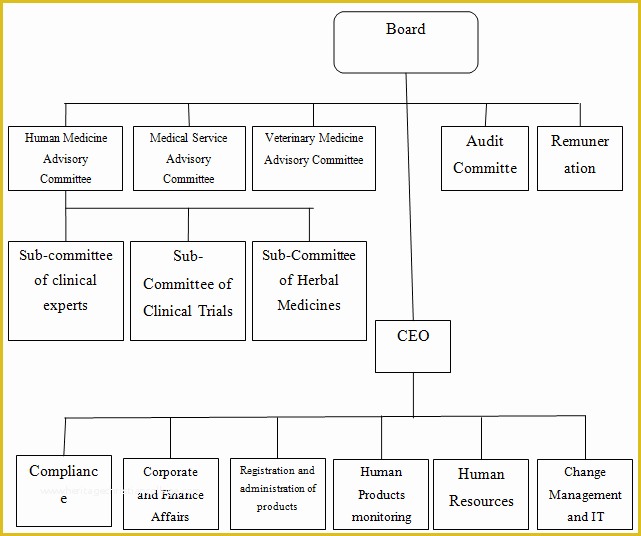
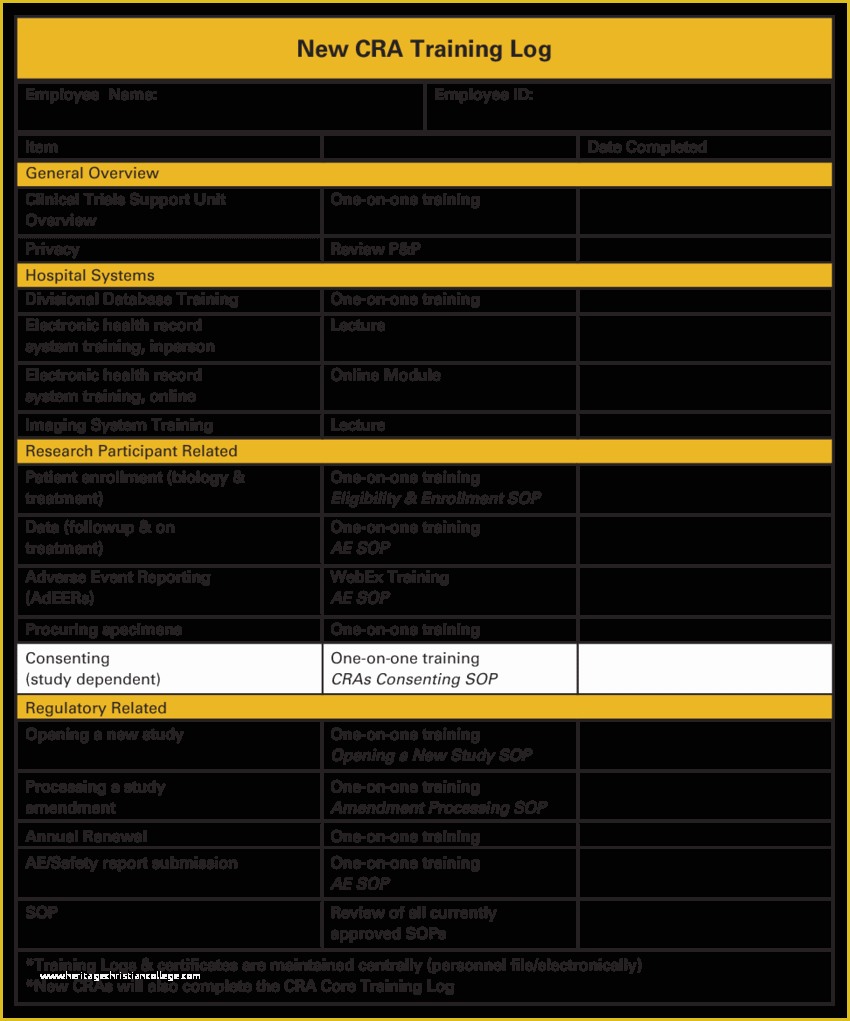
Clinical Pharmacology Fellowship Personal Statement C TA standard operating procedures 20 Free SOP Templates to Make Recording Processes Quick Training log for new clinical research associates CRAs 45 Free SOP Template Description of Pharmacovigilance Audit.
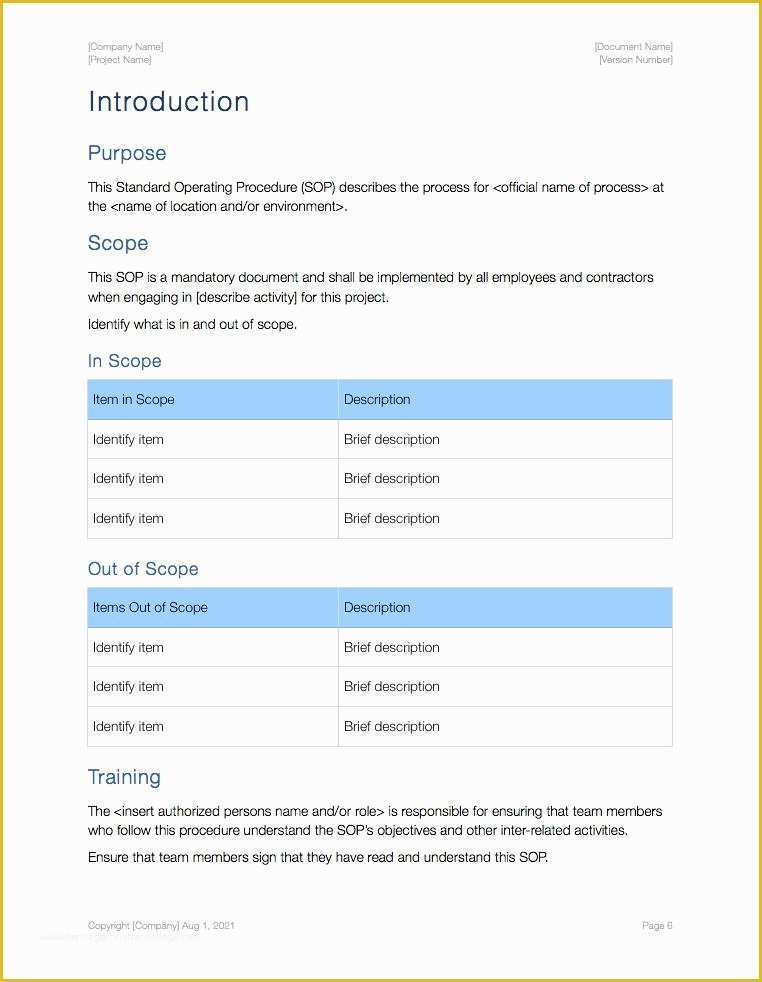
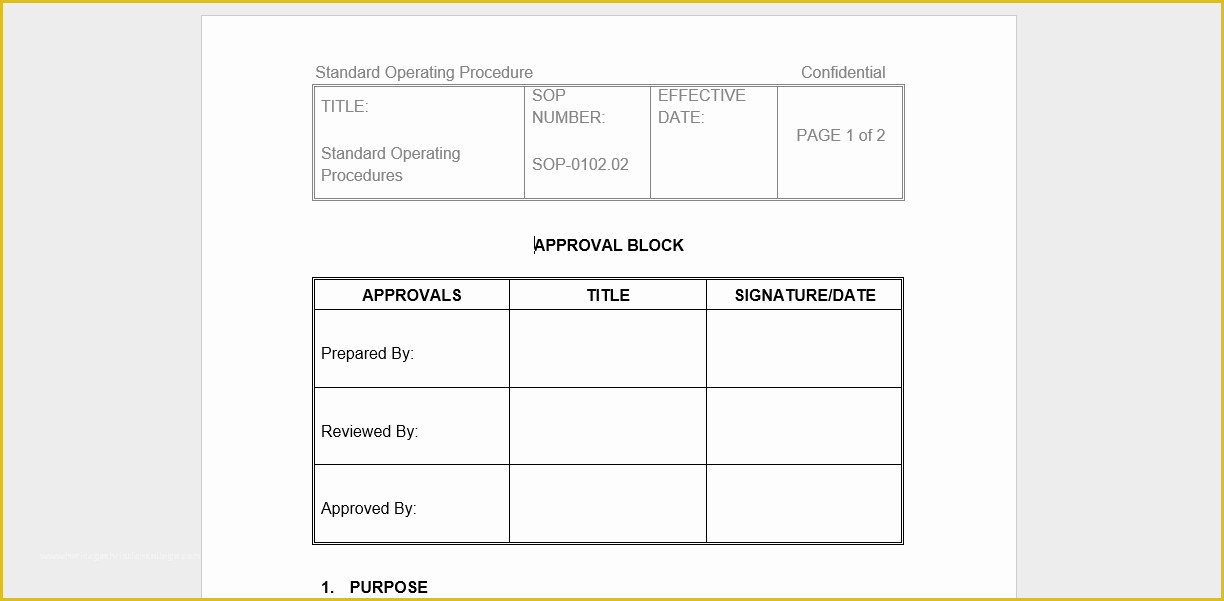
clinical trial clinical trials are experiments or observations done in clinical research such prospective biomedical or behavioral research stu s on human participants are designed to answer specific questions about biomedical or behavioral interventions including new treatments such as novel vaccines s tary choices tary supplements and standard operating procedure template & sop format get free templates and learn from industry practitioners and experts how standard operating procedure sop is used as a pliance tool clinical research acronyms and abbreviations you should how well do you know your clinical research abbreviations here’s a cheat sheet to some mon acronyms and abbreviations that you might encounter research dynamics consulting group resdyncg latest news april 2 2015 – webinar on quality control and your clinical trial lorraine will be providing a free webinar on “building quality control into your clinical trial” on thursday april 2 2015 at 3pm edt 607 free resume templates [download ready made samples resume templates [ready made] get hired for a job position by crafting and sending a well formatted resume to an employer download our free premium resume templates that are ready made and professionally designed consort wel e to the consort website consort stands for consolidated standards of reporting trials and en passes various initiatives developed by the consort group to alleviate the problems arising from inadequate reporting of randomized controlled trials prepare study documentation health research authority protecting and promoting the interests of patients and the public in health research ce3 inc clinical research organization cro clinical research organization free css premium css templates if you can t find a free css website template that suits your needs then why not take a look at the premium templates here standard operating procedure clinical research and practice in clinical research the international council for harmonisation ich defines sops as "detailed written instructions to achieve uniformity of the performance of a specific function"
clinical research unit, clinical research master krems erfahrungen, clinical research in cardiology, clinical research services, clinical research foundation eeu, clinical research meeting, clinical research organisation m nchen, clinical research associate studium, clinical research organisation nrw, clinical research nurse job description,
clinical research organisation berlin, clinical research in cardiology impact factor, clinical research forum, clinical research organisation hamburg, clinical research sops, clinical research specialist, clinical researcher, clinical research mannheim gehalt, clinical research associate studium, clinical research sevices,