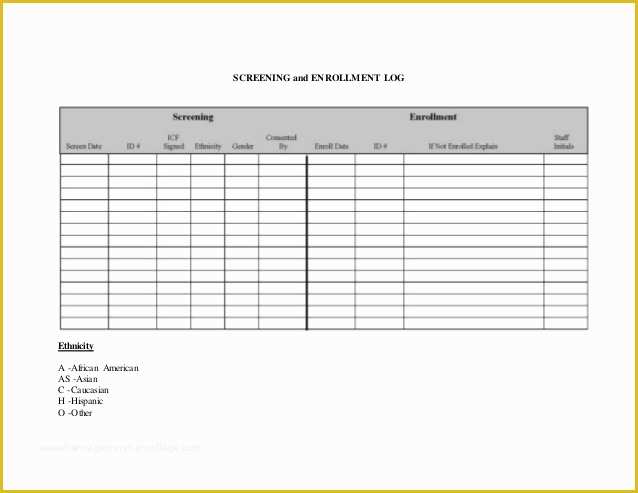
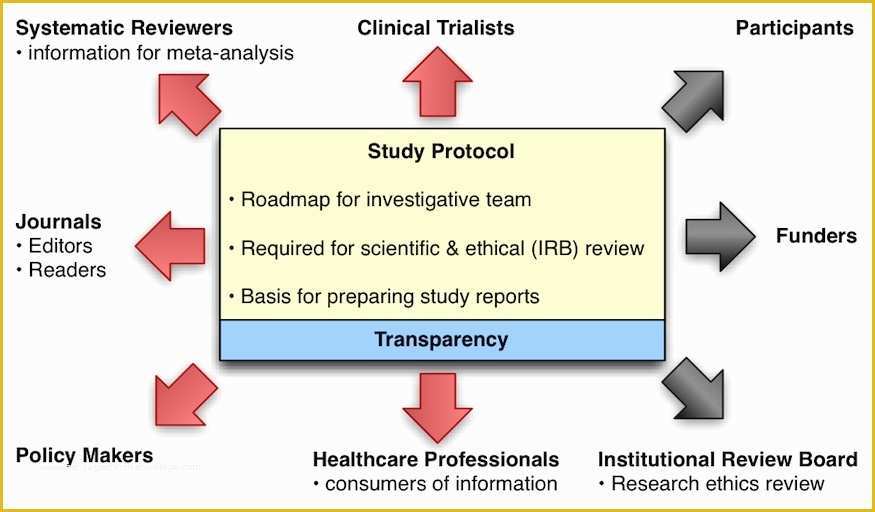
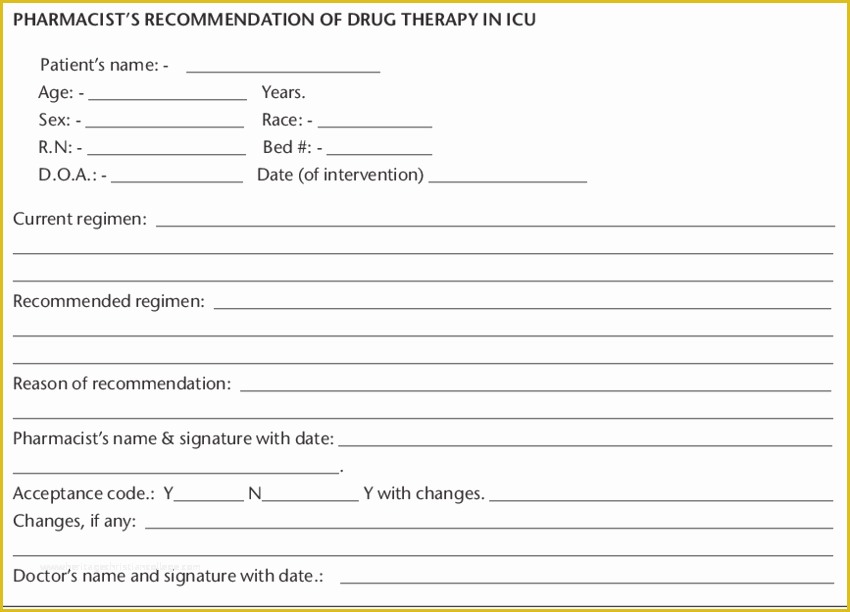
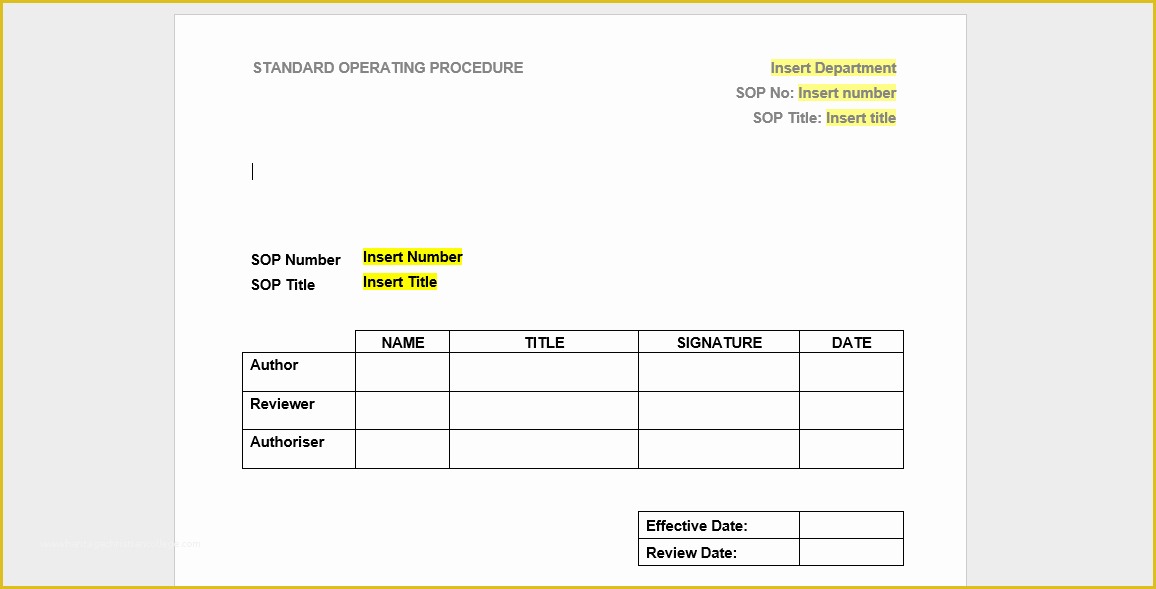
Prepared re mendation form used by the pharmasist Medical History Printable Printables Best 25 Standard operating procedure ideas on Pinterest 36 Page Standard Operating Procedure SOP Template MS Clinical Trial Protocol Template Ich Templates Resume 7 Research Bud Templates Free Sample Example Format.
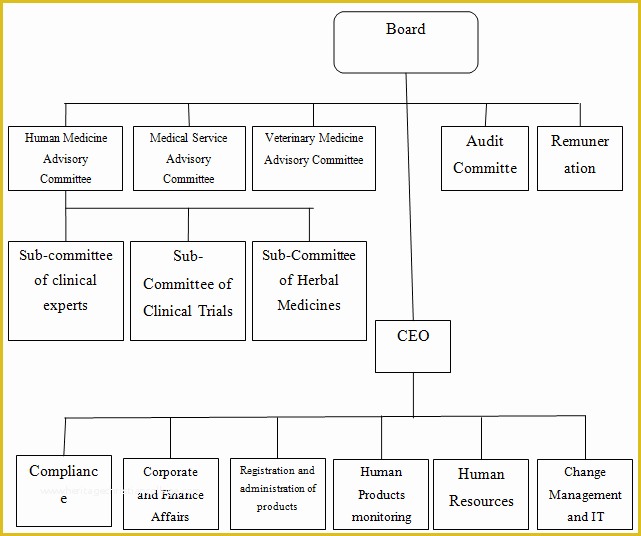
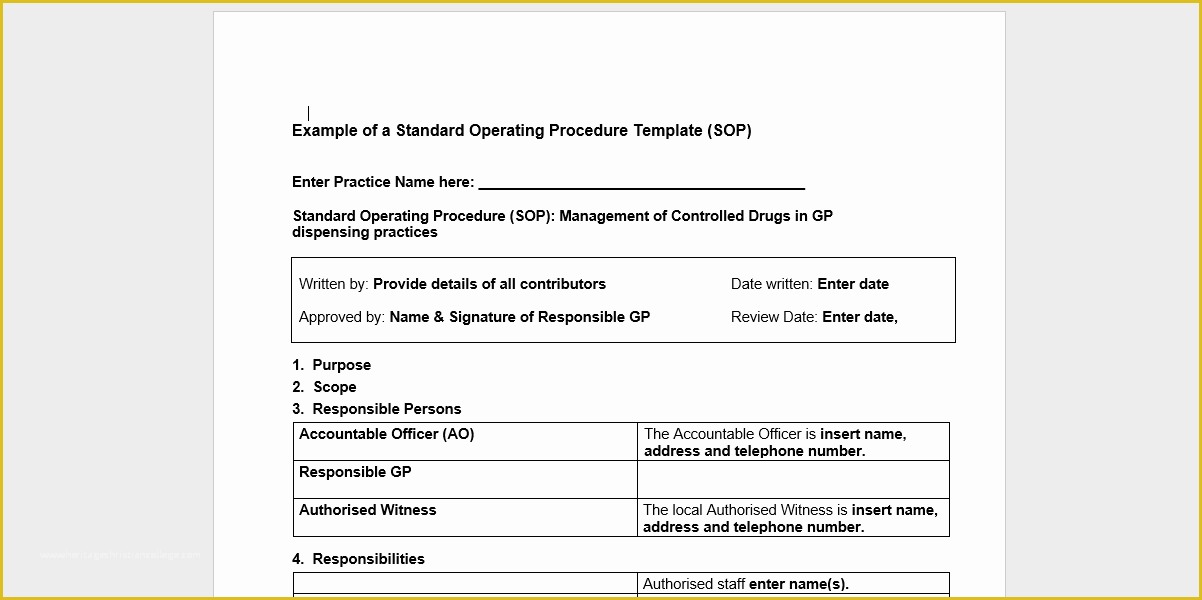
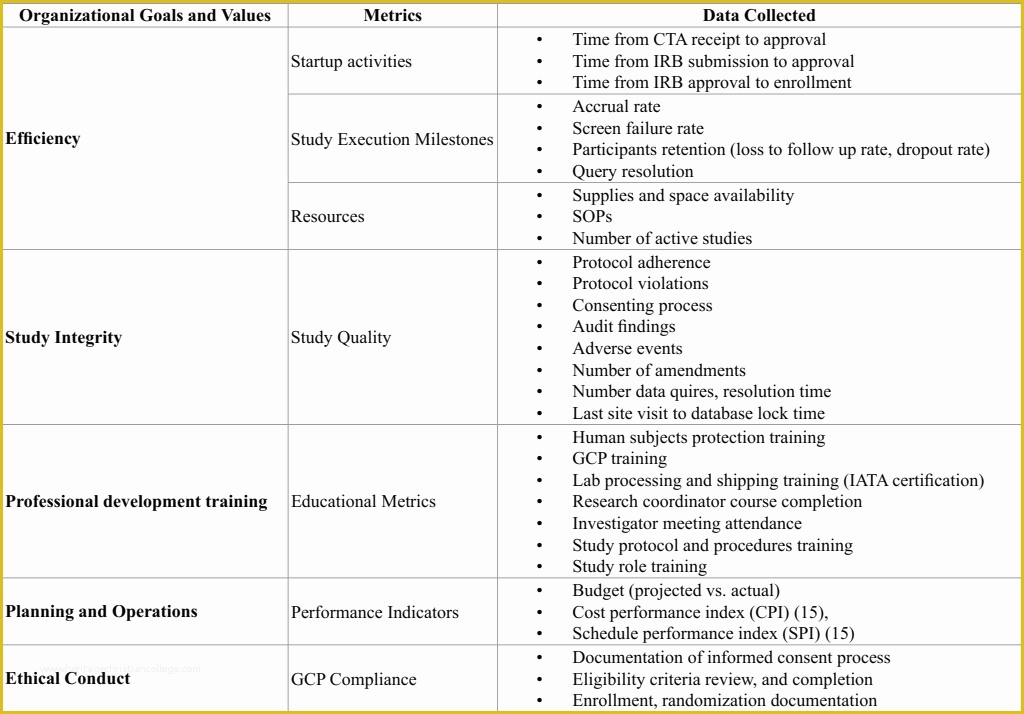
article downloadable templates and tools for clinical wel e to global health trials tools and templates library please note that this page has been updated for 2015 following a quality check and review of the templates and many new ones have been added sops and templates sops and templates standard operating procedures sops are detailed written instructions which must be followed when performing certain tasks they are an essential source for municating to researchers the agreed defined methodology which must be followed to ensure consistency between researchers and in multi centre stu s the consistency between individual research sites standard operating procedures hub clinical research in clinical research sops help define the group’s e g unit division department institution etc standard practices and daily processes conducted to assure execution of research tasks in accordance with institutional state and federal guidances sop writing for clinical research iths sop writing for clinical research write down what you do do what is written down mandy vick research pliance monitor regulatory support & bioethics core 45 free sop template sample templates the best way to design your own standard operating procedure would be to use a free sop template as the framework however if you wish to make your own standard operating procedure without using a template you can follow the steps below standard operating procedures for clinical trials sops standard operating procedures for clinical trials sops dghi is pleased to share these documents with others who are working in resource limited locations although most of these sops were developed for aids clinical trials many of these documents can be modified and extrapolated to meet your specific project needs clinical research gcp sops all clinical research investigators and staff are accountable for following good clinical practice gcp guidelines when conducting human subjects research the college of medicine fice of research has developed gcp clinical research standard operating procedure sop templates which provide detailed written instructions for conducting clinical research at the investigational site standard operating procedures for the conduct of clinical about this sop first published in 1998 standard operating procedures for the conduct of clinical research has be e the industry’s foremost resource for investigative sites seeking to ply with the latest fda regulations and ich gcp guidelines 22 sample sop templates – pdf doc unlike the above this is short and crisp tabular information this also includes other information like date and signature you may also see free sop template 37 best standard operating procedure sop templates how to create a standard operating procedure template by choosing to create a sop template you will be able to standardize your procedures be able to started quickly and you will also be in a position of providing fast and easy to prehend answers to some mon sop questions or queries